Temperature measurement using modern scientific

thermometers and temperature scales goes back at least as far as the early 18th century, when Gabriel Fahrenheit adapted a thermometer (switching to mercury) and a scale both developed by Ole Christensen Rømer. Fahrenheit's scale is still in use in the USA, with the Celsius scale in use in the rest of the world and the Kelvin scale.
Units
The basic unit of temperature (symbol: T) in the International System of Units (SI) is the kelvin (Symbol: K). The kelvin and Celsius scales are, by international agreement, defined by two points: absolute zero, and the triple point of Vienna Standard Mean Ocean Water (water specially prepared with a specified blend of hydrogen and oxygen isotopes). Absolute zero is defined as being precisely 0 K and −273.15 °C. Absolute zero is where all kinetic motion in the particles comprising matter ceases and they are at complete rest in the “classic” (non-quantum mechanical) sense (the relationship between temperature and average kinetic energy is restricted to gases, therefore, it does not apply to temperatures near absolute zero. So zero temperature does not mean that everything is at rest. It means, rather, that all atoms and molecules are in the ground state)[2]. At absolute zero, matter contains no thermal energy. Also, the triple point of water is defined as being precisely 273.16 K and 0.01 °C. This definition does three things: 1) it fixes the magnitude of the kelvin unit as being precisely 1 part in 273.16 parts the difference between absolute zero and the triple point of water; 2) it establishes that one kelvin has precisely the same magnitude as a one degree increment on the Celsius scale; and 3) it establishes the difference between the two scales’ null points as being precisely 273.15 kelvins (0 K = −273.15 °C and 273.16 K = 0.01 °C). Formulas for converting from these defining units of temperature to other scales can be found at Temperature conversion formulas.
In the field of plasma physics, because of the high temperatures encountered and the electromagnetic nature of the phenomena involved, it is customary to express temperature in electronvolts (eV) or kiloelectronvolts (keV), where 1 eV = 11,605 K. In the study of QCD matter one routinely meets temperatures of the order of a few hundred MeV, equivalent to about 1012 K.
For everyday applications, it's very often convenient to use the Celsius scale, in which 0 °C corresponds to the temperature at which water freezes and 100 °C corresponds to the boiling point of water at sea level. In this scale a temperature difference of 1 degree is the same as a 1 K temperature difference, so the scale is essentially the same as the Kelvin scale, but offset by the temperature at which water freezes (273.15 K). Thus the following equation can be used to convert from degrees Celsius to kelvins.
In the United States, the Fahrenheit scale is widely used. On this scale the freezing point of water corresponds to 32 °F and the boiling point to 212 °F. The following conversion formulas may be used to convert between Fahrenheit (F) and Celsius (C) temperature values:
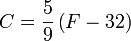 and
and  .
.

![\mathrm{K = [^\circ C] \left(\frac{1 \, K}{1\, ^\circ C}\right) + 273.15\, K}](http://upload.wikimedia.org/math/9/9/1/9912b6ce5daa2c92bc3d79557c739e43.png)


0 comments:
Post a Comment