Heat transfer is the transition of thermal energy from a hotter mass to a cooler mass. When an object is at a different temperature from its surroundings or another object, transfer of thermal energy, also known as heat flow, or heat exchange, occurs in such a way that the body and the surroundings reach thermal equilibrium; this means that they are at the same temperature. Heat transfer always occurs from a higher-temperature object to a cooler-temperature one as described by the second law of thermodynamics or the Clausius statement. Where there is a temperature difference between objects in proximity, heat transfer between them can never be stopped; it can only be decreased.
Conduction
Conduction is the transfer of heat by direct contact of particles of matter. The transfer of energy could be primarily by elastic impact as in fluids or by free electron diffusion as predominant in metals or phonon vibration as predominant in insulators.In other words, heat is transferred by conduction when adjacent atoms vibrate against one another, or as electrons move from one atom to another. Conduction is greater in solids, where a network of relatively fixed spacial relationships between atoms helps to transfer energy between them by vibration.
Heat conduction is directly analogous to diffusion of particles into a fluid, in the situation where there are no fluid currents. This type of heat diffusion differs from mass diffusion in behavior, only in as much as it can occur in solids, whereas mass diffusion is mostly limited to fluids.
Metals (e.g. copper, platinum, gold, iron, etc.) are usually the best conductors of thermal energy. This is due to the way that metals are chemically bonded: metallic bonds (as opposed to covalent or ionic bonds) have free-moving electrons which are able to transfer thermal energy rapidly through the metal.
As density decreases so does conduction. Therefore, fluids (and especially gases) are less conductive. This is due to the large distance between atoms in a gas: fewer collisions between atoms means less conduction. Conductivity of gases increases with temperature. Conductivity increases with increasing pressure from vacuum up to a critical point that the density of the gas is such that molecules of the gas may be expected to collide with each other before they transfer heat from one surface to another. After this point in density, conductivity increases only slightly with increasing pressure and density.
To quantify the ease with which a particular medium conducts, engineers employ the thermal conductivity, also known as the conductivity constant or conduction coefficient, k. In thermal conductivity k is defined as "the quantity of heat, Q, transmitted in time (t) through a thickness (L), in a direction normal to a surface of area (A), due to a temperature difference (ΔT) [...]." Thermal conductivity is a material property that is primarily dependent on the medium's phase, temperature, density, and molecular bonding.
A heat pipe is a passive device that is constructed in such a way that it acts as though it has extremely high thermal conductivity.
- Steady-state conduction vs. Transient conduction
- Steady state conduction is the form of conduction which happens when the temperature difference driving the conduction is constant so that after an equilibration time, the spatial distribution of temperatures (temperature field) in the conducting object does not change any further. For example, a bar may be cold at one end and hot at the other, but the gradient of temperatures along the bar do not change with time. The temperature at any given section of the rod remains constant, and this temperature varies linearly along the direction of heat transfer.
In steady state conduction, the amount of heat entering a section is equal to amount of heat coming out. In steady state conduction, all the laws of direct current electrical conduction can be applied to "heat currents". In such cases, it is possible to take "thermal resistances" as the analog to electrical resistances. Temperature plays the role of voltage and heat transferred is the analog of electrical current.
- Transient conduction There also exists non-steady-state situations, in which the temperature drop or increase occurs more drastically, such as when a hot copper ball is dropped into oil at a low temperature. Here the temperature field within the object changes as a function of time, and the interest lies in analysing this spatial change of temperature within the object over time. This mode of heat conduction can be referred to as transient conduction. Analysis of these systems is more complex and (except for simple shapes) calls for the application of approximation theories, and/or numerical analysis by computer. One popular graphical method involves the use of Heisler Charts.
- Lumped system analysis
A common approximation in transient conduction, which may be used whenever heat conduction within an object is much faster than heat conduction across the boundary of the object, is lumped system analysis. This is a method of approximation that suitably reduces one aspect of the transient conduction system (that within the object) to an equivalent steady state system (that is, it is assumed that the temperature within the object is completely uniform, although its value may be changing in time).
In this method, a term known as the Biot number is calculated, which is defined as the ratio of resistance to heat transfer across the object's boundary with a uniform bath of different temperature, to the conductive heat resistance within the object. When the thermal resistance to heat transferred into the object is less than the resistance to heat being diffused completely within the object, the Biot number less than 1. In this case, particularly for Biot numbers which are even smaller, the approximation of spatially uniform temperature within the object can begin to be used, since it can be presumed that heat transferred into the object has time to uniformaly distribute itself, due to the lower resistance to doing so, as compared with the resistance to heat entering the object.
The Biot number must generally be less than 0.1 for usefully-accurate approximation and heat transfer analysis. The mathematical solution to the lumped system approximation gives Newton's law of cooling, discussed below.
This mode of analysis has been applied to forensic sciences to analyize the time of death ofConvection
| | This section may require cleanup to meet Wikipedia's quality standards. Please improve this section if you can. (March 2009) |
| | This section may require copy editing for grammar, style, cohesion, tone or spelling. You can assist by editing it. (March 2009) |
Convection is the transfer of thermal energy by the movement of molecules from one part of the material to another. As the fluid motion increases, so does the convective heat transfer. The presence of bulk motion of the fluid enhances the heat transfer between the solid surface and the fluid.
There are two types of convective heat transfer:
- Natural convection: when the fluid motion is caused by buoyancy forces that result from the density variations due to variations of temperature in the fluid. For example, in the absence of an external source, when the mass of the fluid is in contact with a hot surface, its molecules separate and scatter, causing the mass of fluid to become less dense. When this happens, the fluid is displaced vertically or horizontally while the cooler fluid gets denser and the fluid sinks. Thus the hotter volume transfers heat towards the cooler volume of that fluid.[3]
- Forced convection: when the fluid is forced to flow over the surface by external source such as fans and pumps, creating an artificially induced convection current.[4]
Internal and external flow can also classify convection. Internal flow occurs when the fluid is enclosed by a solid boundary such as a flow through a pipe. An external flow occurs when the fluid extends indefinitely without encountering a solid surface. Both of these convections, either natural or forced, can be internal or external because they are independent of each other.[citation needed]
The rate of convective heat transfer is given by:[5]
A is the surface area of heat transfer. Ts is the surface temperature and Tb is the temperature of the fluid at bulk temperature. However, Tb varies with each situation and is the temperature of the fluid “far” away from the surface. h is the constant heat transfer coefficient that depends upon physical properties of the fluid such as temperature and the physical situation in which convection occurs. Therefore, the heat transfer coefficient must be derived or found experimentally for every system analyzed. Formulas and correlations are available in many references to calculate heat transfer coefficients for typical configurations and fluids. For laminar flows, the heat transfer coefficient is rather low compared to the turbulent flows; this is due to turbulent flows having a thinner stagnant fluid film layer on heat transfer surface.[6]
Radiation
Radiation is the transfer of heat energy through empty space. All objects with a temperature above absolute zero radiate energy at a rate equal to their emissivity multiplied by the rate at which energy would radiate from them if they were a black body. No medium is necessary for radiation to occur, for it is transferred through electromagnetic waves; radiation works even in and through a perfect vacuum. The energy from the Sun travels through the vacuum of space before warming the earth.
Both reflectivity and emissivity of all bodies is wavelength dependent. The temperature determines the wavelength distribution of the electromagnetic radiation as limited in intensity by Planck’s law of black-body radiation. For any body the reflectivity depends on the wavelength distribution of incoming electromagnetic radiation and therefore the temperature of the source of the radiation. The emissivity depends on the wave length distribution and therefore the temperature of the body itself. For example, fresh snow, which is highly reflective to visible light, (reflectivity about 0.90) appears white due to reflecting sunlight with a peak energy wavelength of about 0.5 micrometres. Its emissivity, however, at a temperature of about -5°C, peak energy wavelength of about 12 micrometres, is 0.99.
Gases absorb and emit energy in characteristic wavelength patterns that are different for each gas.
Visible light is another form of electromagnetic radiation with a shorter wavelength (and therefore a higher frequency) than infrared radiation. The difference between visible light and the radiation from objects at conventional temperatures is a factor of about 20 in frequency and wavelength; the two kinds of emission are simply different "colors" of electromagnetic radiation.
Clothing and building surfaces, and radiative transfer
Lighter colors and also whites and metallic substances absorb less illuminating light, and thus heat up less; but otherwise color makes little difference as regards heat transfer between an object at everyday temperatures and its surroundings, since the dominant emitted wavelengths are nowhere near the visible spectrum, but rather in the far infrared. Emissivities at those wavelengths have little to do with visual emissivities (visible colors); in the far infrared, most objects have high emissivities. Thus, except in sunlight, the color of clothing makes little difference as regards warmth; likewise, paint color of houses makes little difference to warmth except when the painted part is sunlit. The main exception to this is shiny metal surfaces, which have low emissivities both in the visible wavelengths and in the far infrared. Such surfaces can be used to reduce heat transfer in both directions; an example of this is the multi-layer insulation used to insulate spacecraft. Low-emissivity windows in houses are a more complicated technology, since they must have low emissivity at thermal wavelengths while remaining transparent to visible light.
humans. Also it can be applied to HVAC (heating, ventilating and air-conditioning, or building climate control), to ensure more nearly instantaneous effects of a change in comfort level setting.
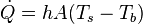


0 comments:
Post a Comment